RESEARCH SUPPORT
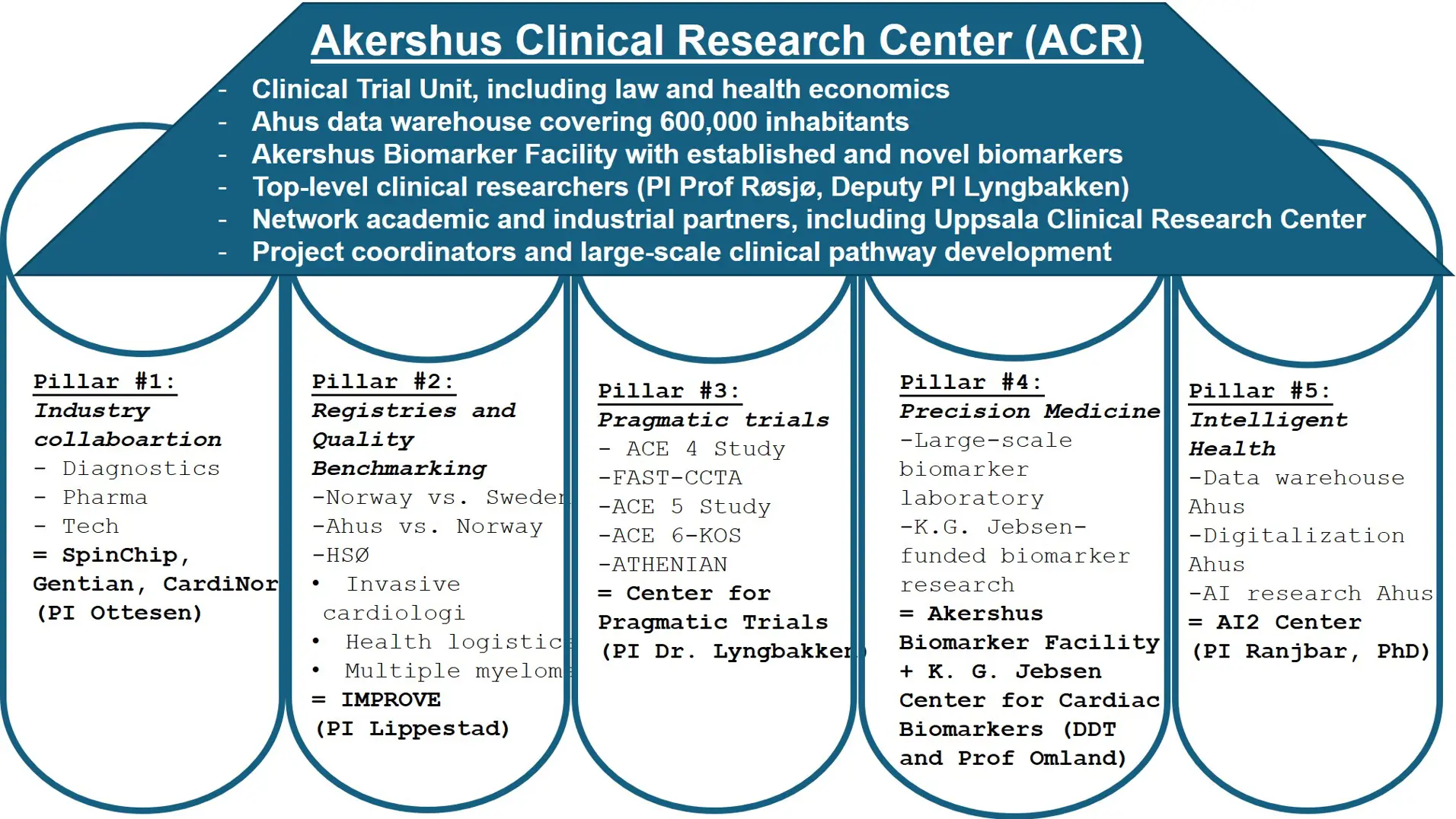
Akershus Clinical Research Center (ACR)
ACR helps you where you are - from idea to protocol, execution and publication.
Research support at Akershus University Hospital (Ahus) offers support and advice to you as a researcher throughout the entire research process.
ACR strengthens this offer with advisers who provide assistance where academic resources are needed. We support studies at Ahus in all subject areas: from idea to protocol, implementation and publication.
We mainly assist researchers at Ahus, but can also help others by agreement.
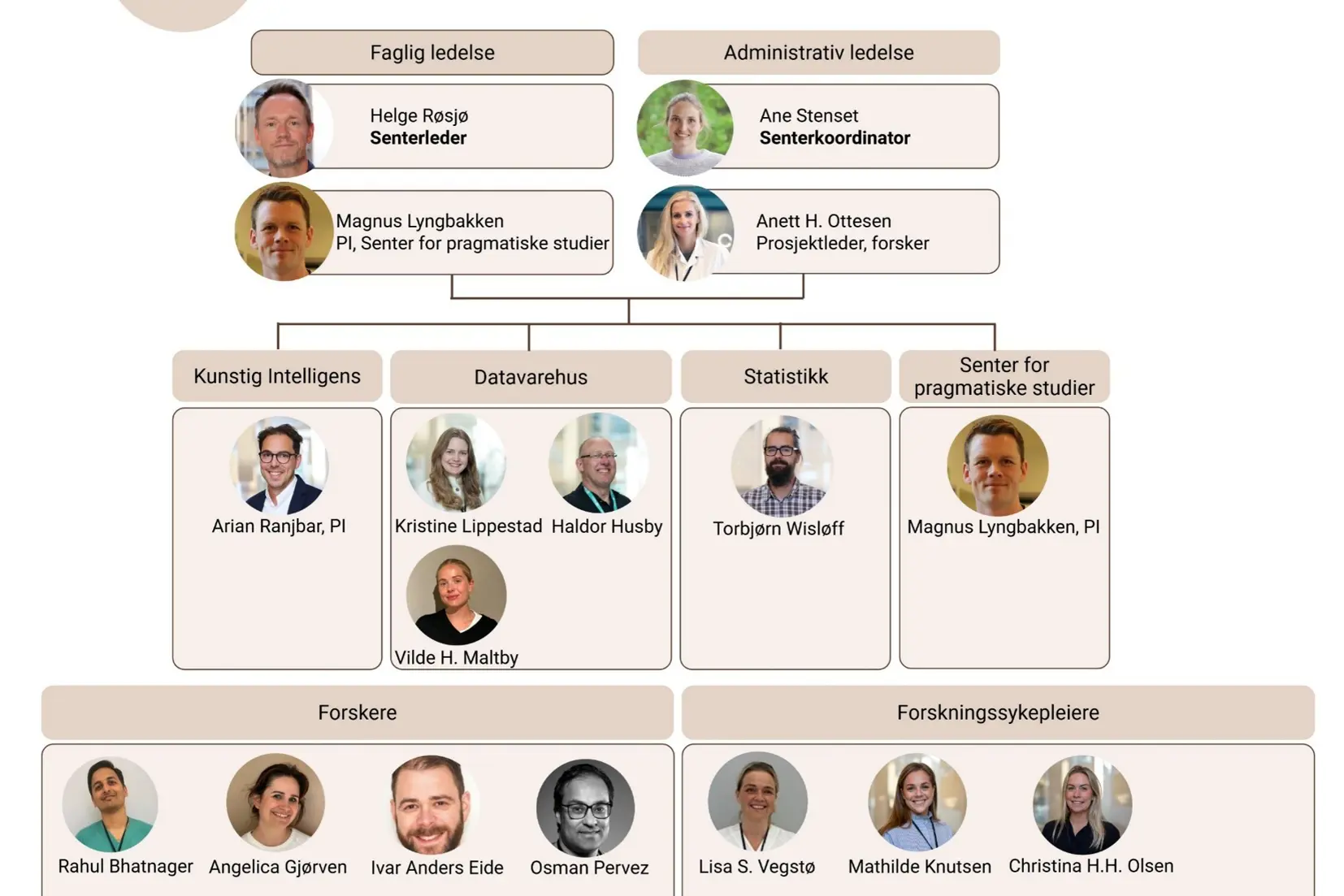
ACR consists of
- Research support with strong expertise and demonstrable ability to implement within researcher-initiated clinical studies, including support in privacy and health economics.
- Ahu's data warehouse covering over 605,000 inhabitants.
- Sustainable and reliable AI.
- Laboratory analyses of established and new biomarkers.
- Infrastructure led by active top researchers.
- Registries and quality benchmarking: Benchmarking Quality of Cardiovascular Care (PDF)
ACR collaborates with the following research centres
Pragmatic clinical trials use clinical registries and data systems to randomize patients in routine clinical practice, aiming to establish real-world evidence using patients in real-life clinical settings. In Sweden, Uppsala Clinical Research Center has demonstrated the great potential of pragmatic trials using Swedish national quality registries as the platform, resulting in several landmark papers in The New England Journal of Medicine. Unfortunately, due to no formal collaboration, Norway has not contributed to these important trials. In 2023, we established the Centre for Pragmatic Clinical Trials at Akershus University Hospital. The centre leader, Associate Professor Magnus Nakrem Lyngbakken, is a leading young researcher in Norway related to pragmatic trials, already being responsible for the pragmatic NO COVID-19 Study, which led to two publications in Nature Communication. Lyngbakken was until recently Head of the working group for pragmatic trials in the Norwegian Clinical Research Infrastructures Network (NorCRIN), which is a collaboration between all teaching hospitals in Norway related to clinical trials, with a funding of 50 MNOK from the Research Council of Norway in 2020. The centre aims to deliver high-quality clinical trials directly influencing patient outcomes and decision-making.
https://clinicaltrials.gov/study/NCT04316377
Abstract
The hypothesis of the study is that treatment with hydroxychloroquine sulphate in hospitalised patients with coronavirus disease 2019 (Covid-19) is safe and will accelerate the virological clearance rate for patients with moderately severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) when compared to standard care. We randomized 53 patients hospitalized with coronavirus disease 2019 (COVID-19) to hydroxychloroquine therapy (at a dose of 400 mg twice daily for seven days) in addition to standard care or standard care alone. All severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) positive patients 18 years of age or older were eligible for study inclusion if they had moderately severe COVID-19 at admission. Treatment with hydroxychloroquine did not result in a significantly greater rate of decline in SARS-CoV-2 oropharyngeal viral load compared to standard care alone during the first five days. Our results suggest no important antiviral effect of hydroxychloroquine in humans infected with SARS-CoV-2. Data from the NO COVID-19 study was later merged with international data on hydroxychloroquine therapy in patients with SARS-CoV-2, and this meta-analysis demonstrated that treatment with hydroxychloroquine was associated with increased mortality in Covid-19 patients.
References
Lyngbakken et al. Norwegian Coronavirus Disease 2019 (NO COVID-19) Pragmatic Open label Study to assess early use of hydroxychloroquine sulphate in moderately severe hospitalised patients with coronavirus disease 2019: A structured summary of a study protocol for a randomised controlled trial. Trials. 2020;21:485 (https://doi.org/10.1186/s13063-020-04420-0)
Lyngbakken et al. A pragmatic randomized controlled trial reports lack of efficacy of hydroxychloroquine on coronavirus disease 2019 viral kinetics. Nat Commun. 2020;11:5284 (https://doi.org/10.1038/s41467-020-19056-6)
Axfors et al. Mortality outcomes with hydroxychloroquine and chloroquine in COVID-19 from an international collaborative meta-analysis of randomized trials. Nat Commun. 2021;12:2349 (https://doi.org/10.1038/s41467-021-22446-z)
https://clinicaltrials.gov/study/NCT05045612
Abstract
Antimicrobial resistance is one of the most urgent health threats of our time, and Norwegian hospitals were required to reduce the use of broad-spectrum antibiotics with 30% by the end of 2020. In the ATHENIAN study, we aim to assess the efficacy and safety of early discontinuation of antibiotic therapy in adult patients infected with respiratory viruses. We hypothesize that discontinuation of antibiotic therapy in patients with an airway sample positive for respiratory viruses is safe and non-inferior to continuation of antibiotic therapy. Patient inclusion is ongoing in multiple Norwegian study sites, with an expected completion of patient inclusion during 2025. The results of the study hold potential for implementation in several areas of clinical practice. Antimicrobial resistance is a global health threat, and clinical trials aiming at reducing antibiotic usage will directly alleviate this challenge. Increased knowledge on over-usage of antibiotic therapy will help advice decision makers in establishing the national antibiotic programs, and accordingly improve national and international strategies related to management and therapy in a considerable proportion of acutely ill patients in need of hospital admission.
https://clinicaltrials.gov/study/NCT05699564
Abstract
Patients hospitalized with tachypnea, defined as respiratory rate ≥20/min, have substantial mortality and may suffer from different conditions, including acute heart failure (HF). Symptoms of HF can be difficult to identify and approximately 15% of patients with HF will not be correctly diagnosed by the treating physician in the Emergency Department. Measurement of biomarkers like B-type natriuretic peptides (BNPs) and cardiac troponins may improve diagnostic accuracy and risk stratification. Whether early, structured biomarker assessment and structured feedback in the patient's electronic health records improve management and outcomes among unselected patients with tachypnea has not been explored in a randomized controlled trial. The study is a single-center, pragmatic randomized controlled trial of a non-pharmacological intervention, and patients will be randomized to (1) early biomarker measurements and structured feedback or (2) standard of care. The main research question of the study is to determine whether early structured biomarker assessment in unselected patients with tachypnea extends the time to the first event for either (1) all-cause readmission or (2) all-cause mortality, compared to standard of care, at follow-up after 12 months. Patient inclusion in the ACE4 study is ongoing and the last patient is expected to be recruited during the Spring of 2024.
Abstract
Patient injuries and adverse events are recognized as main challenges in the Norwegian National Action Plan for Patient Safety and Quality Improvement. The Norwegian Health Directory´s patient safety program "In safe hands 24/7" recommends huddle board as a tool to monitor patient safety measures, as defined in the program. There is limited information whether huddle boards impact clinical and patient safety outcomes. In addition, electronic health records now provide opportunity to integrate data for individual patients and report these back in structured format to clinical personnel on large screens, so-called Patient Safety Monitors (PSMs). In the ACT1 study, we hypothesize that the use of PSMs in hospital wards in a Norwegian Orthopedic Department will reduce hospital length of stay and improve clinical and patient safety outcomes. The study is a single-center, pragmatic, pseudo-randomized controlled trial with in silico-control group for a non‑pharmacological intervention. Results publised: https://doi.org/10.1371/journal.pone.0335052
Abstract
Patients undergoing operative treatment for colorectal cancer have 20-30% risk of suffering major complication after surgery. Early post-operative complications are associated with poor long-term outcome and increases expenditures. There have only been small modifications in surgical techniques during the last decade. Focus on pre-operative education, training, and dietary habits (prehabilitation) have gained attention and could improve patient outcome. The hypothesis for prehabilitation is that a multimodal prehabilitation program prior to colorectal cancer surgery will reduce length of stay, postoperative complications and reduce mortality in colorectal cancer patients. In this single-center, pseudo-randomized controlled trial we will assess whether patients surgically treated for colorectal cancer have better clinical outcomes after a standardized prehabilitation program compared to patients surgically treated for colorectal cancer without a prehabilitation program. This project might bring new knowledge of importance for individual patients and for the health system. Puplication planned spring 2026.
- NorTrials Cardiovascular (NorTrials.no)
- KG Jebsen Center for Cardiac Markers (uio.no)
- Uppsala Clinical Research Center
Center leader
Helge Røsjø: Helge.rosjo@ahus.no
Telephone : 91 54 58 64
Magnus Nakrem Lyngbakken: m.n.lyngbakken@medisin.uio.no
Coordinators
Ane Stenset: Ane.stenset@ahus.no
Telephone: 41 63 02 11
Karin A. Vassbakk: Karin.vassbakk@ahus.no